-
Mar 6, 2024
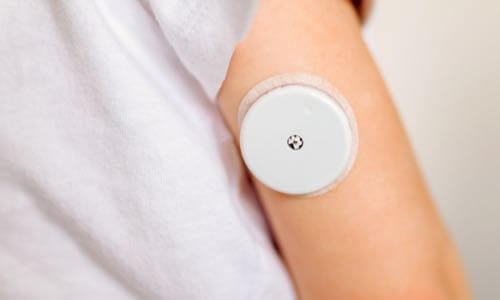
Stelo by Dexcom, the first glucose biosensor cleared by the FDA for over-the-counter use, will be available in the US for individuals aged 18 and older not using insulin therapy, making it accessible without a prescription. This small, wearable sensor, worn on the upper arm, will transmit glucose data directly to the user's smartphone. Developed by Dexcom, Stelo aims to empower people with Type 2 diabetes to manage their health effectively. Available from summer 2024 onwards, Stelo fills a crucial gap in the market, providing continuous glucose monitoring for those without insurance coverage or prescription access.
-
Feb 20, 2024
Modular Medical, Inc. has submitted its MODD1 next-generation insulin pump for FDA clearance, aiming to revolutionize the diabetes market with user-friendly and affordable technology. Chairman Paul DiPerna highlights the company's goal of encouraging wider adoption of insulin pumps by simplifying design and reducing costs.
-
Jan 22, 2024
The latest version of the CGM Certificate Program was built on our new learning platform. Enhancements range from more active engagement through interactive elements to more flexibility and accessibility.
-
Jan 12, 2024
Dexcom unveiled its new Stelo device during the J.P. Morgan Healthcare Conference. The Stelo is based on Dexcom's G7 CGM and is specifically designed for individuals who do not take insulin. This product is part of Dexcom's ongoing efforts to cater to a broader range of patients.
-
Dec 7, 2023
Tandem Diabetes Care, Inc. has announced the release of updated t:slim X2™ insulin pump software featuring integration with Dexcom G7 Continuous Glucose Monitoring (CGM) technology in the United States. The company plans to provide a complimentary remote software update to all in-warranty t:slim X2 users, allowing them to access Dexcom's latest CGM advancements. New t:slim X2 pumps with the integrated software are currently being shipped to customers, while the Dexcom G7 integration is expected to launch in additional countries outside the U.S. in early 2024.
-
Nov 16, 2023
AI is a tool to amplify human expertise, not replace it. Its footprint in diabetes technology is already substantial and growing rapidly. Like everyone, healthcare professionals need to stay informed and even, embrace AI's role in diabetes care for better patient outcomes.
-
Nov 16, 2023
Insulet Corporation has received FDA clearance for its Omnipod 5 App for iPhone, becoming the first company to offer a tubeless automated insulin delivery system with complete control via compatible Android and iOS smartphones. It will integrate with the Dexcom G6 Continuous Glucose Monitoring System initially, with a full market release in 2024, and is available at no cost to Omnipod 5 System users.
-
Nov 16, 2023
Dexcom is in the process of discontinuing Dexcom G6. They are working in phases to transition all of their customers to Dexcom G7 and stand-alone Dexcom G6 systems are the first phase.
-
Nov 16, 2023
The tubeless Accu-Chek Solo micropump system from Roche has gained FDA approval, providing a new insulin delivery solution for individuals aged 2 years and older with type 1 diabetes.
-
Nov 16, 2023
Tandem Diabetes Care announced a tech access program to give t:slim X2 customers a purchase pathway to the tandem mobi system for $199.
-
Nov 16, 2023
ADCES 2023 is full of amazing educational sessions, exhibitors, and opportunities for hands-on learning with a tech focus and since I don’t want to bury the lead, this year we also get puppies!
-
Nov 16, 2023
Tandem Diabetes Care, Inc. (NASDAQ: TNDM), a leading insulin delivery and diabetes technology company, today announced U.S. Food and Drug Administration (FDA) clearance for the Tandem Mobi insulin pump for people with diabetes age 6 and up, expanding the company’s portfolio of products. The Tandem Mobi is fully controllable from a mobile app and is the world’s smallest durable automated insulin delivery system.
-
Nov 16, 2023
A recent study published in The Lancet reveals that the global prevalence of diabetes is expected to more than double in the next 30 years, affecting over 1.3 billion people worldwide. Currently, over half a billion people are living with diabetes, with a global prevalence rate of 6.1%. Diabetes is identified as one of the top 10 leading causes of death and disability globally.
The study highlights regional variations, with the highest rates found in North Africa and the Middle East. Additionally, the research emphasizes that almost all cases (96%) are type 2 diabetes (T2D), with high body mass index (BMI) being the primary risk factor. The study emphasizes the complex nature of diabetes prevention and control, involving genetic, logistical, social, and financial factors. The findings stress the need for a comprehensive understanding of diabetes impact at a granular level to address disparities and improve access to screening and treatment. The study was supported by the Bill & Melinda Gates Foundation.
-
Nov 16, 2023
One educator’s “lessons learned” on working with new technology and upgrades.
-
Nov 16, 2023
The FDA has cleared the iLet Bionic Pancreas system for patients aged 6 and older with type 1 diabetes. The pocket-sized system includes the Beta Bionics iLet ACE pump and the iLet Dosing Decision Software, along with an integrated continuous glucose monitor (CGM). The FDA's decision was based on the system's Breakthrough Device Designation and aims to provide the type 1 diabetes community with more options for diabetes management. The system is easy to initiate, as it only requires the patient's body weight for insulin dosing and does not need manual adjustments. It features a meal announcement feature that estimates carb consumption, removing the need for traditional carb counting. The system demonstrated its efficacy in a pivotal trial, showing improved HbA1c levels and increased time spent in the target glucose range.
-
Nov 16, 2023
American Association of Clinical Endocrinology (AACE) has released the 2023 Comprehensive Type 2 Diabetes Management Algorithm to guide healthcare professionals in clinical decision-making for the management of Type 2 diabetes. The updated algorithm emphasizes a person-centered approach to care, incorporating the latest evidence-based recommendations, associated conditions, complications, and health equity. Key elements include lifestyle modification and treatment of overweight and obesity, treatment of cardiovascular risk factors, and immunization recommendations for people with T2D.
-
Nov 16, 2023
Insulet Corporation has announced the FDA clearance of its latest innovation, Omnipod GO, an insulin delivery device cleared for use for people with type 2 diabetes age 18 or older who would typically take daily injections of long-acting insulin. Omnipod GO is a standalone, wearable, insulin delivery system that provides a fixed rate of continuous rapid-acting insulin for 72 hours.
The newest addition to the Omnipod brand features a tubeless and waterproof Pod which is offered in seven different pre-programmed daily rates, ranging from 10 to 40 units per day, and operates without the need for a handheld device to control the Pod. The product was developed as an alternative to daily injections. Insulet plans to commercialize Omnipod GO in the United States in 2024.
-
Nov 16, 2023
Eli Lilly and Company (NYSE: LLY) and Amphastar Pharmaceuticals, Inc. (NASDAQ: AMPH) have entered into a definitive agreement for Lilly to divest BAQSIMI worldwide to Amphastar, a global pharmaceutical company focused on developing, manufacturing, and marketing injectable, intranasal, and inhalation products including experience with a glucagon product. BAQSIMI is the first and only nasally administered glucagon for the treatment of severe hypoglycemia in people with diabetes.
Amphastar expects to provide dedicated commercial investment for BAQSIMI with the goal of enabling more people on insulin to be prepared with a glucagon rescue treatment for severe hypoglycemia.
-
Nov 16, 2023
Medtronic has received US FDA approval for its MiniMed 780G system with the Guardian 4 sensor, a closed-loop insulin delivery system for people living with type 1 diabetes. The system, which features meal detection technology, provides automatic adjustments and corrections to sugar levels for both basal and bolus insulin needs. It is the only system on the market with a glucose target setting as low as 100 mg/dL, which more closely mirrors the average glucose of someone without diabetes. Users can wear the infusion set for up to seven days, double the wear time of similar systems. The MiniMed 780G system is approved for people aged seven and above with type 1 diabetes.
-
Apr 12, 2023
Aspect Biosystems, and Novo Nordisk are collaborating to develop bioprinted tissue therapeutics for the treatment of diabetes and obesity. Novo Nordisk will have an exclusive worldwide license to use Aspect's bioprinting technology to develop up to four products to treat diabetes and/or obesity. The goal of the collaboration is to develop implantable bioprinted tissues that will replace, repair, or supplement biological functions inside the body to deliver a new class of disease-modifying treatments. The collaboration will initially focus on developing bioprinted tissue therapeutics designed to maintain normal blood glucose levels without the need for immunosuppression, which could represent a transformative treatment for people living with type 1 diabetes.


 Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.
Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.-(3).jpg?sfvrsn=16486d59_5)






 Find danatech's interactive diabetes technology tools for point-of-care, insurance coverage and more.
Find danatech's interactive diabetes technology tools for point-of-care, insurance coverage and more.